In order to ensure the quality of the product, the company has established a high level quality inspection center and set up a team. Our products are mainly used in medicine, dietary supplement, cosmetics and other fields with the functions of oxidation resistance, delaying ageing, improving blood glucose, etc. We provide customers with all-round professional personalized solutions and services.


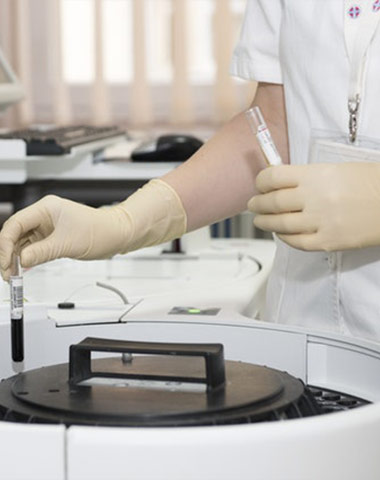
The company carry out complete quality management with quality manual under
GMP regulation.
For API, we have obtained GMP certificate and passed site inspection by FDA.



The enterprise guides with information, guarantee with technology and win the market with quality is the management philosophy of the enterprise. It is our motivation to cooperate with customers both at home and abroad.
 Transparent pricing and full-disclosure supply chain available
Transparent pricing and full-disclosure supply chain available
 Continuous development and supply of new, science-based ingredients
Continuous development and supply of new, science-based ingredients
 Nutraceutical distribution of amino acids, vitamins and specialty actives
Nutraceutical distribution of amino acids, vitamins and specialty actives
 Contract and blanket order pricing available
Contract and blanket order pricing available
 Responsible for providing test reports.
Responsible for providing test reports.
 Any time for you to service.
Any time for you to service.
 Technical product documentation
Technical product documentation
 USA warehousing for fast delivery
USA warehousing for fast delivery
